Not long until the Joint Clinical Assessment begins: Will PICO anxiety become a reality?

Are you ready?
Recent developments
Scientific specifications of medicinal products
New active substance |
A new chemical, biological, or radiopharmaceutical active substance that:
|
Cancer treatment |
|
ATMP |
|
Key: ATMP – advanced therapy medicinal product; EC – European Commission; EU – European Union; HTACG – Health Technology Assessment Coordination Group; ICD-O –International Classification of Diseases for Oncology; JCA – Joint Clinical Assessment.
Outstanding implementing acts and guidance documents
Implementing act |
Deadline |
Status |
|
Procedural rules for JCA of medicinal products |
Q1–Q2 2024 |
Adopted 23 May 2024 |
|
Rules on cooperation by exchange of information with the EMA |
Q3 2024 |
Adopted 18 October 2024 |
|
Procedural rules for the management of conflict of interest |
Q3 2024 |
Adopted 25 October 2024 |
|
Procedural rules for JSC of medicinal products |
Q3 2024 |
Final version in preparation, public consultation closed |
|
Procedural rules for JSC of medical devices and IVD medical devices |
Q4 2024 |
Public consultation open until 26 November 2024 |
|
Procedural rules for JCA of medical devices and IVD medical devices |
Q4 2024 |
Planned |
Key: EMA – European Medicines Agency; IVD – in vitro diagnostic; JCA – Joint Clinical Assessment; JSC – Joint Scientific Consultation.
Additionally, more than 10 guidance documents are currently in preparation and supposed to be published before the JCA comes into effect. Among these, the guidance describing the scientific methodology for the scoping process is particularly eagerly awaited by stakeholders since it is expected to complement previous preliminary guidance by EUnetHTA 21 on how this ‘first step’ of the JCA process, such as the definition of the PICO (population, intervention, comparator, outcome) for the JCA scope, will be conducted.
Initiation of the JCA process by the health technology developer

PICO definition
Table 3. Comparison of PICO simulations in mCRPC by EUnetHTA 21 and Cencora
|
|
EUnetHTA 21 |
Cencora |
|
Indication |
Adult patients with progressive prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy |
|
|
Number of Member States covered |
8 (Countries not specified) |
16 (Belgium, Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal, Spain, and Sweden) |
|
Results (no.) |
|
|
|
Populations |
Full licensed population + 4 subpopulations |
Full licensed population + 4 subpopulations |
|
Intervention |
Pluvicto |
Cenpicomab |
|
Comparators |
6 |
12 |
|
Outcomes |
21 |
20 (see Figure 1) |
|
Consolidated PICOs |
6, including 2 for the full licensed population |
18, including 9 for the full licensed population (see Table 4) |
|
Date of PICO survey |
November 2022 |
May 2024 |
Key: no. – number; PICO – population, intervention, comparator, outcome.
The comparison of both simulations shows that including a broader range of Member States and extending the timeline to capture the most recently approved comparators may lead to a significant increase in consolidated PICOs to be considered for the JCA scope due to variations in clinical guidelines, treatment standards, and speed of market access of new medicines across EU countries.
Comparators
According to EUnetHTA 21’s practical guideline, every required comparator across the included countries results in a separate PICO for the assessment scope. Following this practical guideline, Cencora’s analysis led to a high number in PICOs for the full population driven by the requirement of five of the 16 countries (Germany, Ireland, Italy, Portugal, and Spain) to include all locally relevant comparators in the clinical assessment, covering a range of two comparators in Italy to seven comparators in Portugal. In contrast, three countries (Czech Republic, France, and the Netherlands) stated that assessment against one locally relevant comparator was sufficient, another country (Poland) stated that the relevant comparator would be selected based on the highest market share, and five countries (Belgium, Denmark, Finland, Norway, and Sweden) highlighted that the relevant treatment option would depend on the positioning of the product (i.e. the line of treatment) by the HTD. In Luxembourg, the assessment is based on the dossier submitted in the official ‘pays de provenance’ (i.e. country of origin, usually Belgium). In Greece, only one comparator was considered relevant for the assessment scope. Table 4 shows the identified PICOs for the full population of cenpicomab.
For the EUnetHTA 21 analysis, country-specific requirements have not been published, reflecting the scoping process in the framework of the JCA, where individual country responses will not be made available to HTDs.
|
|
Comparators of interest for the full population for cenpicomab |
Countries requesting each comparator |
PICO 1 |
Abiraterone + prednisone/prednisolone |
BE, DE, FI, FR, IE, LU, NL, NO, PT, SE |
PICO 2 |
Best supportive care* |
DE, DK, ES, FI, IE, NL, NO, SE |
PICO 3 |
Cabazitaxel + prednisone/prednisolone* |
BE, DE, DK, ES, FI, IE, IT, LU, NL, NO, PT, SE |
PICO 4 |
Docetaxel + prednisone/prednisolone* |
BE, DK, FI, IE, LU, NL, NO, PT, SE |
PICO 5 |
Enzalutamide |
BE, DE, DK, FI, FR, IE, LU, NL, NO, PT, SE |
PICO 6 |
Lutetium (177Lu) vipivotide tetraxetan + androgen deprivation therapy* |
BE, DE, FI, GR, IE, IT, NL, PO, PT |
PICO 7 |
Olaparib* |
DK, PT |
PICO 8 |
Radium-223* |
ES, FI, PO |
PICO 9 |
Radium-223 + luteinizing hormone releasing hormone analogue* |
PO, PT |
Key: BE – Belgium; CZ – Czech Republic; DE – Germany; DK – Denmark; ES – Spain; FI – Finland; FR – France; GR – Greece; IE – Ireland; IT – Italy; LU – Luxembourg; NL – Netherlands; NO – Norway; PICO – population, intervention, comparator, outcome; PO – Poland; PT – Portugal; SE – Sweden.
Note: Countries in bold represent the countries the PICO was derived from due to the request for data against all identified comparators.
*Comparators also considered in PICOs for subpopulations (not presented here).
The possibility of extensive PICOs resulting from the scoping phase was confirmed in a recent EFPIA simulation for three authorised oncology treatments, including orphan and ATMP, which resulted in a large number of potential PICOs across seven countries, ranging from seven to 23 PICOs after consolidation.
Outcomes
Figure 1. Number of countries requesting data against specified clinical outcomes
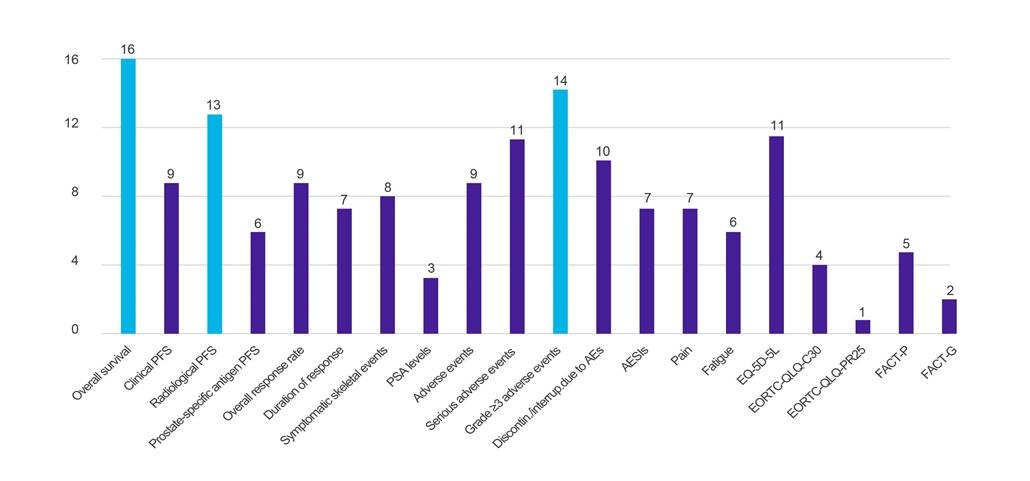
Key: AE – adverse event; AESI – adverse event of special interest; EORTC-QLQ-C30 – European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (-Core, 30-item); EORTC-QLQ-PR25 – European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (-Prostate, 25-item); EQ-5D-5L – EuroQol-5 Dimension-5 Level; FACT-G – Functional Assessment of Cancer Therapy-General; FACT-P – Functional Assessment of Cancer Therapy-Prostate; PFS – progression-free survival; PSA – prostate-specific antigen.
Conclusions

With less than two months remaining until the JCA comes into effect, and with methodological guidance still pending, significant uncertainties persist for both the industry and HTA bodies regarding the scoping process, which forms the basis of the JCA. PICO exercises organised by the HTACG and involving various HTA bodies, as well as PICO simulations conducted by HTDs, are crucial for preparing key stakeholders for the upcoming JCA in terms of capability and internal strategy. However, it remains to be seen how these simulations will translate into the actual JCA and what impact the assessment will have on local market access. The EU HTA will function as a learning system during the initial JCAs, and the collaboration, effort, and feedback of all stakeholders will be essential to create an efficient system.
Sources
-
EUnetHTA 21. Practical Guideline. D4.2 Scoping process. Version 1.0. 2022. Accessed 23 September 2024. https://www.eunethta.eu/wp-content/uploads/2022/09/EUnetHTA-21-D4.2-practical-guideline-on-scoping-process-v1.0.pdf
-
EUnetHTA 21. Consolidated PICO. PICO exercise I – Pluvicto. Version 1.0. 2023. Accessed 23 September 2024. https://www.eunethta.eu/wp-content/uploads/2023/09/EUnetHTA-21-PICO-1-Deliverbale-1.pdf
-
European Commission. Regulation (EC) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on Health Technology Assessment and Amending Directive 2011/24/EU. 2021. Accessed 23 September 2024. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 11 May 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-05-11_en
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 18 September 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-09-18_en
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 22 November 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-11-22_en
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 30 January 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-01-30_en
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 9 April 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-04-09_en
-
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 23 September 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-09-06_en
-
European Commission. Implementation rolling plan 2023-2024. Updated September 2024. Accessed 23 September 2024. https://health.ec.europa.eu/document/download/397b2a2e-1793-48fd-b9f5-7b8f0b05c7dd_en
-
European Commission. Health technology assessment – joint clinical assessments of medical devices. 2024. Accessed 5 November 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13707-Health-technology-assessment-joint-clinical-assessments-of-medical-devices_en
-
European Commission. Health technology assessment – joint clinical assessments of medicinal products. 2024. Accessed 24 September 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13708-Health-technology-assessment-joint-clinical-assessments-of-medicinal-products_en
-
European Commission. Health technology assessment – procedural rules for assessing and managing conflicts of interest. 2024. Accessed 23 September 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13751-Health-technology-assessment-procedural-rules-for-assessing-and-managing-conflicts-of-interest_en
-
European Commission. Health technology assessment – cooperation with the European Medicines Agency. 2024. Accessed 23 September 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/14164-Health-technology-assessment-cooperation-with-the-European-Medicines-Agency_en
-
European Commission. Health technology assessment – joint scientific consultations on medical devices & in vitro diagnostic medical devices. 2024. Accessed 5 November 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13752-Health-technology-assessment-joint-scientific-consultations-on-medical-devices-in-vitro-diagnostic-medical-devices_en
-
European Commission. Health technology assessment – joint scientific consultations on medicinal products for human use. 2024. Accessed 22 October 2024. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13759-Health-technology-assessment-Joint-scientific-consultations-on-medicinal-products-for-human-use_en
-
European Commission. Joint clinical assessment of medicinal products: submission of early information by health technology developers. 2024. Accessed 23 September 2024. https://health.ec.europa.eu/document/download/77476507-9ddc-47e3-ae0d-11c05f89169c_en?filename=hta_%20mp-jca_htd_en.pdf
-
European Federation of Pharmaceutical Industries and Associations. EU HTA Regulation for oncology medicines: Learnings from a simulation on the impact of proposed EUnetHTA21 methods. 2024. Accessed 23 September 2024. https://www.efpia.eu/media/qrjah2ij/efpia-evidera-research-on-eunethta21-methods.pdf
-
European Medicines Agency. Advanced therapy medicinal products: Overview. 2024. Accessed 23 September 2024. https://www.ema.europa.eu/en/human-regulatory-overview/advanced-therapy-medicinal-products-overview
-
HTACG. Scientific specifications of medicinal products subject to joint clinical assessments. 2024. Accessed 23 September 2024. https://health.ec.europa.eu/document/download/48974c78-1c37-4cf9-9fc9-a630fee9baac_en?filename=hta_mp_jca_sc-specifications_en.pdf
-
O’Donnell R. From PICO anxiety to PICO strategy: planning for EU HTA success. 2024. Accessed 23 September 2024. https://www.amerisourcebergen.com/insights/manufacturers/from-pico-anxiety-to-pico-strategy




