Article
Navigating indirect comparisons in health technology assessments: Insights from German AMNOG evaluations
In the intricate world of health technology assessment, indirect comparisons are often indispensable resources for evaluating new pharmaceuticals when direct evidence is not available. This article delves into the rigorous landscape of German AMNOG evaluations, revealing key insights and priorities that shape the future of healthcare innovation.
An essential first step in the HTA process is to demonstrate that the methodology used to generate comparative data vs. the ACT is appropriate. If the Federal Joint Committee (G-BA) accepts the methodology, all data are taken into account in the added benefit assessment. However, if an IC is not accepted, these data are disregarded, and the assessment of added benefit relies on direct evidence alone.
The acceptance criteria for ICs are stringent, with success often hinging on special circumstances. Methodological limitations, such as heterogeneity across studies and inherent biases in the evidence on the comparator side, can introduce uncertainty to the comparative assessments. It is essential for researchers and evaluators to transparently address these limitations and employ robust methodologies to enhance the validity and reliability of IC results. Methods guidance regarding the requirements for ICs is provided by the Institute for Quality and Efficiency in Health Care (IQWiG). In addition, drug developers can schedule an additional advice meeting with the G-BA to gain further insights on the appropriate methodology. This article explores the findings from recent analyses of ICs in German AMNOG assessments, highlighting the stringent criteria for acceptance and the implications for various therapeutic fields.
The acceptance criteria for ICs are stringent, with success often hinging on special circumstances. Methodological limitations, such as heterogeneity across studies and inherent biases in the evidence on the comparator side, can introduce uncertainty to the comparative assessments. It is essential for researchers and evaluators to transparently address these limitations and employ robust methodologies to enhance the validity and reliability of IC results. Methods guidance regarding the requirements for ICs is provided by the Institute for Quality and Efficiency in Health Care (IQWiG). In addition, drug developers can schedule an additional advice meeting with the G-BA to gain further insights on the appropriate methodology. This article explores the findings from recent analyses of ICs in German AMNOG assessments, highlighting the stringent criteria for acceptance and the implications for various therapeutic fields.
Methods
A systematic search for ICs was performed using an internal AMNOG database containing every benefit assessment published on the website of the G-BA until April 2024. The assessment of the ICs by the G-BA and the associated added medical benefits were obtained from the database. This comprehensive analysis included 222 completed benefit assessments encompassing 334 subpopulations.
Results
Among the 334 subpopulations for which an IC was presented, the predominant therapeutic fields were oncology (51.2%), metabolic (15.0%), and infectious (11.4%) diseases (Figure 1). In 22.5% of the cases, the IC was accepted by the G-BA (Figure 2). Methodological deficiencies and insufficient similarity of the compared studies were the main reasons for the rejection of an IC by the G-BA (Figure 3).
Figure 1. ICs in German benefit assessments by therapeutic field
The G-BA’s rigorous criteria ensure that only robust and reliable ICs are accepted. This emphasis on methodological rigor is crucial for maintaining the integrity of HTA processes. As a rule, the G-BA requires adjusted comparisons, such as the IC according to Bucher. However, both adjusted and unadjusted comparisons were identified among the accepted ICs, whereas other methods, such as network meta-analyses or propensity score matching, were not considered methodologically appropriate. Consequently, the database analysis showed that unadjusted comparisons may also be deemed acceptable under certain circumstances, such as in highly vulnerable populations or when facing significant challenges within the therapeutic field.
Figure 2. Acceptance of ICs by the G-BA
For instance, in the case of chronic hepatitis C virus infection, interferon-containing combination therapies were defined as ACT prior to 2014. Interferons are associated with significant side effects, including depression and suicidal thoughts. Given the treatment success that can be achieved with the newer drugs sofosbuvir and elbasvir/grazoprevir, along with the potential for shorter therapy durations and the avoidance of severe interferon-related side effects, it was deemed necessary for ethical reasons to consider uncontrolled, single-arm studies in which these newer drugs were compared with historical controls. Thus, an adjusted comparison was not feasible, and, in this case, an unadjusted comparison was accepted by the G-BA.
Special considerations are also given to highly vulnerable populations, such as pediatric patients or those with rare diseases. In those cases, RCTs may not be ethically feasible to conduct. In the context of lysosomal acid lipase deficiency, for instance—a rare disease that typically results in increased mortality within the first year of life with no available treatment alternatives at the time of assessment—the manufacturer provided ICs with historical controls to overcome the lack of direct comparative evidence. The G-BA accepted this methodology and concluded that the results of the IC ruled out the possibility that the demonstrated gain in overall survival could only be explained by methodological uncertainty. Therefore, the G-BA granted a hint of a non-quantifiable additional benefit to sebelipase alfa. This is an example of the acceptance of an unadjusted IC under additional exceptional circumstances.
Figure 3. Reasons for rejection of ICs by the G-BA
Figure 1. ICs in German benefit assessments by therapeutic field
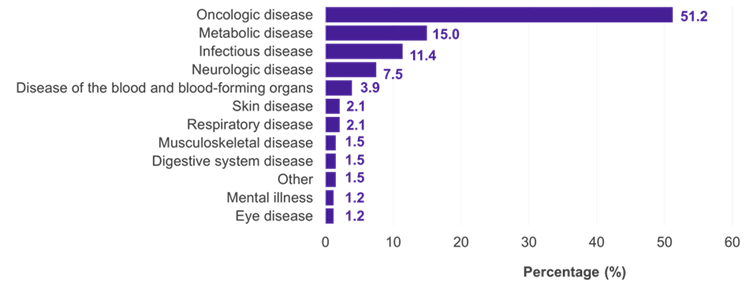
Key: IC – indirect comparison.

The G-BA’s rigorous criteria ensure that only robust and reliable ICs are accepted. This emphasis on methodological rigor is crucial for maintaining the integrity of HTA processes. As a rule, the G-BA requires adjusted comparisons, such as the IC according to Bucher. However, both adjusted and unadjusted comparisons were identified among the accepted ICs, whereas other methods, such as network meta-analyses or propensity score matching, were not considered methodologically appropriate. Consequently, the database analysis showed that unadjusted comparisons may also be deemed acceptable under certain circumstances, such as in highly vulnerable populations or when facing significant challenges within the therapeutic field.
Figure 2. Acceptance of ICs by the G-BA
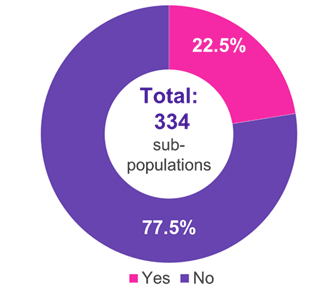
Key: G-BA – Federal Joint Committee; IC – indirect comparison.

For instance, in the case of chronic hepatitis C virus infection, interferon-containing combination therapies were defined as ACT prior to 2014. Interferons are associated with significant side effects, including depression and suicidal thoughts. Given the treatment success that can be achieved with the newer drugs sofosbuvir and elbasvir/grazoprevir, along with the potential for shorter therapy durations and the avoidance of severe interferon-related side effects, it was deemed necessary for ethical reasons to consider uncontrolled, single-arm studies in which these newer drugs were compared with historical controls. Thus, an adjusted comparison was not feasible, and, in this case, an unadjusted comparison was accepted by the G-BA.
Special considerations are also given to highly vulnerable populations, such as pediatric patients or those with rare diseases. In those cases, RCTs may not be ethically feasible to conduct. In the context of lysosomal acid lipase deficiency, for instance—a rare disease that typically results in increased mortality within the first year of life with no available treatment alternatives at the time of assessment—the manufacturer provided ICs with historical controls to overcome the lack of direct comparative evidence. The G-BA accepted this methodology and concluded that the results of the IC ruled out the possibility that the demonstrated gain in overall survival could only be explained by methodological uncertainty. Therefore, the G-BA granted a hint of a non-quantifiable additional benefit to sebelipase alfa. This is an example of the acceptance of an unadjusted IC under additional exceptional circumstances.
Figure 3. Reasons for rejection of ICs by the G-BA
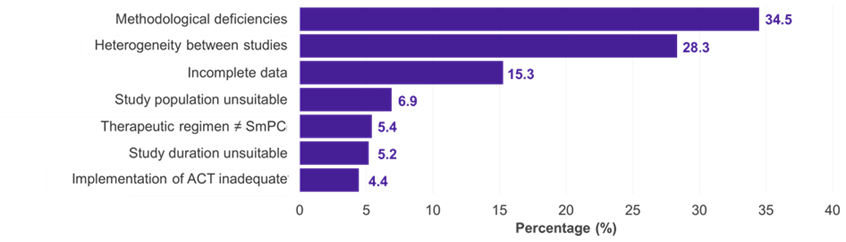
Key: ACT – appropriate comparator therapy; G-BA – Federal Joint Committee; IC – indirect comparison; SmPC – summary of product characteristics.


Conclusion
In conclusion, the analysis of ICs within the framework of German AMNOG assessments highlights the complexity and high standards required for such evaluations. The stringent criteria set by the G-BA ensure that only robust and methodologically sound ICs are accepted, emphasising the necessity of transparent and rigorous methodological approaches to ensure the validity and reliability of results. Although only a small percentage of ICs are ultimately accepted, ICs will continue to provide critical evidence in specific therapeutic contexts such as vulnerable populations.
The findings illustrate that under certain circumstances, adjusted and unadjusted comparisons can be accepted, particularly when ethical considerations preclude direct head-to-head trials, and treatment of patients with rare or severe diseases is prioritised. In summary and from the HTA point of view, it is first advisable to carefully assess the feasibility to conduct RCTs. In cases where an RCT is not feasible, ICs are a suitable method to compensate for the lack of direct comparative data, but the choice of an appropriate methodology is critical. To maximise the likelihood of acceptance in the German context, it is advisable to adhere closely to the strict criteria of the G-BA.
Authors

Health technology assessments (HTAs) are essential for determining the value and impact of new pharmaceuticals on healthcare systems. In Germany, the AMNOG (Pharmaceuticals Market Reorganisation Act) process requires pharmaceutical companies to demonstrate the medical benefit of newly approved drugs against an appropriate comparator therapy (ACT). To obtain an added medical benefit, the data must demonstrate an advantage of the assessed drug over the ACT beyond a set threshold. While randomised controlled trials (RCTs) are the gold standard for evidence, indirect comparisons (ICs) often become necessary when direct head-to-head comparisons are unfeasible.
Sources
-
Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683-691. https://dx.doi.org/10.1016/s0895-4356(97)00049-8
-
Federal Joint Committee (G-BA). 2014. Frühe Nutzenbewertung: Beträchtlicher Zusatznutzen für Wirkstoff gegen chronische hepatitis C. Accessed 31 October 2024. https://www.g-ba.de/presse/pressemitteilungen-meldungen/546/
-
Federal Joint Committee (G-BA). 2017. AM-RL-XII Elbasvir-Grazoprevir. Accessed 31 October 2024. https://www.g-ba.de/downloads/40-268-4429/2017-06-15_AM-RL-XII_Elbasvir-Grazoprevir_D-268_TrG.pdf
-
Federal Joint Committee (G-BA). 2021. AM-RL-XII Sebelipase alfa. Accessed 31 October 2024. https://www.g-ba.de/downloads/40-268-7570/2021-06-03_AM-RL-XII_Sebelipase-alfa_D-606_TrG.pdf
-
Federal Joint Committee (G-BA). 2024. Benefit assessments database. Accessed 31 October 2024. https://www.g-ba.de/
-
Institute for Quality and Efficiency in Health Care (IQWiG). 2023. General Methods Version 7.0. 19 September 2023. https://www.iqwig.de/methoden/general-methods_version-7-0.pdf
-
Warmbold B, Scharrenbroich J, Teich K, Ingendoh-Tsakmakidis A, Löpmeier-Röh J, Kulp W. Indirect comparisons in German AMNOG assessments: keep the faith. Presented at: ISPOR Annual European Congress; Barcelona, Spain; 2024.
This article summarises Cencora’s understanding of the topic based on publicly available information at the time of writing (see listed sources) and the authors’ expertise in this area. Any recommendations provided in the article may not be applicable to all situations and do not constitute legal advice; readers should not rely on the article in making decisions related to the topics discussed.





