PDABs: An imperfect solution to a complex issue
Andrew York, Executive Director MD PDAB
Katie Chandra, Senior Director and Head of State Policy at Genentech
Tiffany Westrich-Robertson, CEO and founder of International Foundation for Autoimmune & Autoinflammatory Arthritis (AiArthritis), lead of Ensuring Access through Collaborative Health (EACH), and coordinator of Patient Inclusion Council (PIC)

An imperfect system of Prescription Drug Affordability Boards may deliver the promise of lower-cost medicines to patients, but is there room to improve?
The pricing of prescription drugs in the United States

At the state level, some have enacted legislation to allow pharmaceutical imports from Canada, while 11 states have enacted legislation to form Prescription Drug Affordability Boards (PDABs).

An introduction to PDABs
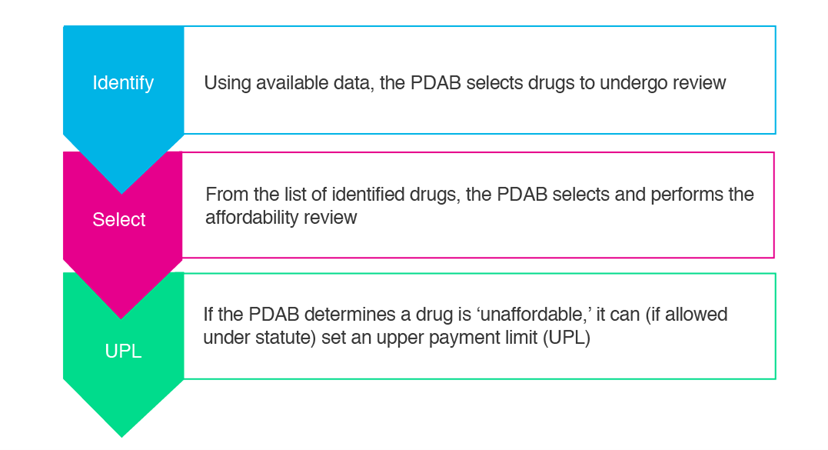
PDABs are proposed as a mechanism to control spending on prescription medicines, which are deemed ‘unaffordable’ for patients and state purchasers. The first PDAB was introduced in 2019 by the Maryland legislature. The National Academy for State Health Policy outlined a process for PDABs as described in the diagram below.
The goal: Allow access to the right drugs for patients who need them—it’s simple, right?
PDABs have proven to be very challenging to implement, and none has yet reached the stage of generating savings for patients, states, or health systems. Implementation challenges create opportunities for process improvement in PDAB approaches to prescription drug selection and affordability reviews. During the ISPOR 2025 meeting in Montreal, an Issue Panel discussed the perceived issues that PDABs present, and three areas that present immediate opportunities for the development of best practices were outlined: 1) clearly defining affordability and PDAB goals; 2) data collection and analysis; and 3) meaningful stakeholder engagement.
1. Clearly defining affordability and PDAB goals
2. Data collection and analysis
3. Meaningful stakeholder engagement
If advertisements around PDABs claim to focus on reducing costs for patients, then they should be designed to allow for improved and meaningful patient engagement, and outcomes should address patient-reported needs. However, current methods for collecting input from patients vary from PDAB to PDAB; similar questions lacked meaningful input, and, in turn, these methods may not reflect their lived experience or context related to potential affordability challenges.
Furthermore, a narrative was introduced early on that patient advocacy organisations should not be trusted solely on the premise that most get some of their funding from pharmaceutical companies. As a result, some PDABs have dismissed their expertise, while elevating credibility to others who represent patient voices and receive funding from other entities. This perspective is not only frustrating to organisations, but it is potentially damaging to the process of increasing patient engagement.
State PDABs could serve stakeholders better if they could collaborate to develop and share the best practices, which would make processes more predictable, reproducible, and transparent. Patient engagement should be a key component of these efforts, with PDABs investing in question design development that could be used across boards and that are robust enough to capture the ‘why’ behind affordability successes and challenges. The benefit of PDABs to patients will only be realised when patients are at the center of a process that is designed to benefit them.
This article summarises Cencora’s understanding of the topic based on publicly available information at the time of writing (see listed sources) and the authors’ expertise in this area. Any recommendations provided in the article may not be applicable to all situations and do not constitute legal advice; readers should not rely on the article in making decisions related to the topics discussed. Panelists' opinions are their own and do not necessarily reflect those of their respective employers.
Connect with our team

Sources
- Pharmapproach. Why are prescription drugs so expensive in the U.S.? Latest 2025 updates on drug pricing, Medicare negotiations, and cost-saving solutions. March 26, 2024. Accessed 15 August 2025. Available at: https://www.pharmapproach.com/why-are-prescription-drugs-so-expensive-in-the-u-s-latest-2025-updates-on-drug-pricing-medicare-negotiations-and-cost-saving-solutions/
- Fick A, Shalal A, Graham D. Exclusive: US pharma tariffs likely weeks away as Trump plans for Alaska, sources say. Reuters. August 13, 2025. Accessed 15 August 2025. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/us-pharma-tariffs-likely-weeks-away-trump-plans-alaska-sources-say-2025-08-13/
- The White House. Fact sheet: President Donald J. Trump announces actions to get Americans the best prices in the world for prescription drugs. July 31, 2025. Accessed 15 August 2025. Available at: https://www.whitehouse.gov/fact-sheets/2025/07/fact-sheet-president-donald-j-trump-announces-actions-to-get-americans-the-best-prices-in-the-world-for-prescription-drugs/



