Discover more
Improve inspection-readiness
Our TMF quality review (sometimes called a “file review”) provides a line-by-line evaluation of your TMF, giving you detailed insight into its quality, completeness, and timeliness. Much more than just a series of TMF data reports, the customer-proven review is conducted by our expert TMF practitioners with deep understanding of current inspection standards at each major regulatory agency.
Drawing on our global leadership in Trial Master File best practices and experience conducting hundreds of TMF quality reviews, our proven process helps ensure your TMF meets good clinical practice (GCP) standards and regulatory requirements:
Drawing on our global leadership in Trial Master File best practices and experience conducting hundreds of TMF quality reviews, our proven process helps ensure your TMF meets good clinical practice (GCP) standards and regulatory requirements:
Analysis
Comprehensive analysis of individual studies as well as trend analysis across studies.
Heatmaps
Innovative, purpose-built tools such as TMF heatmaps.
Risk-based assessments
Risk-based assessments to quickly pinpoint and drill down to problem areas.
Quality checks
Logic checks and cross-checks to validate the quality of individual documents and whether documents all relate to each other properly.
Compatibility with eTMF Systems
Flexibility to be used with any eTMF system in addition to Cencora’s advanced eTMF software, PhlexTMF.
Expert recommendations
Detailed, expert recommendations for necessary remediation.
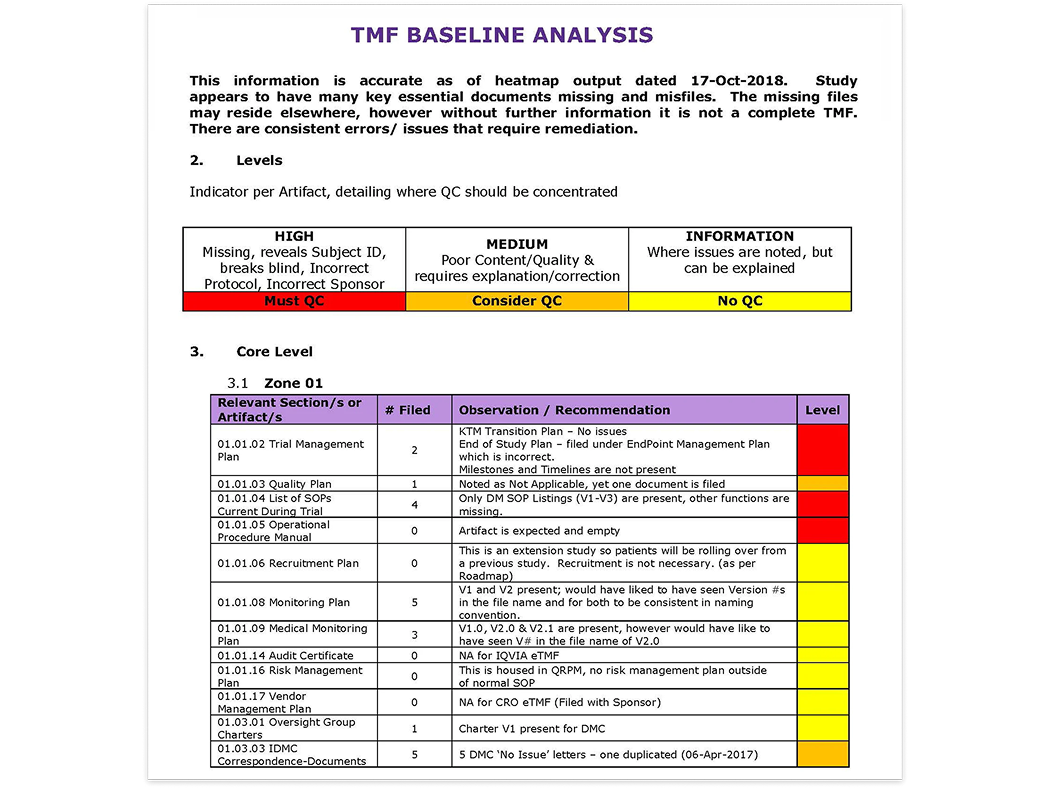
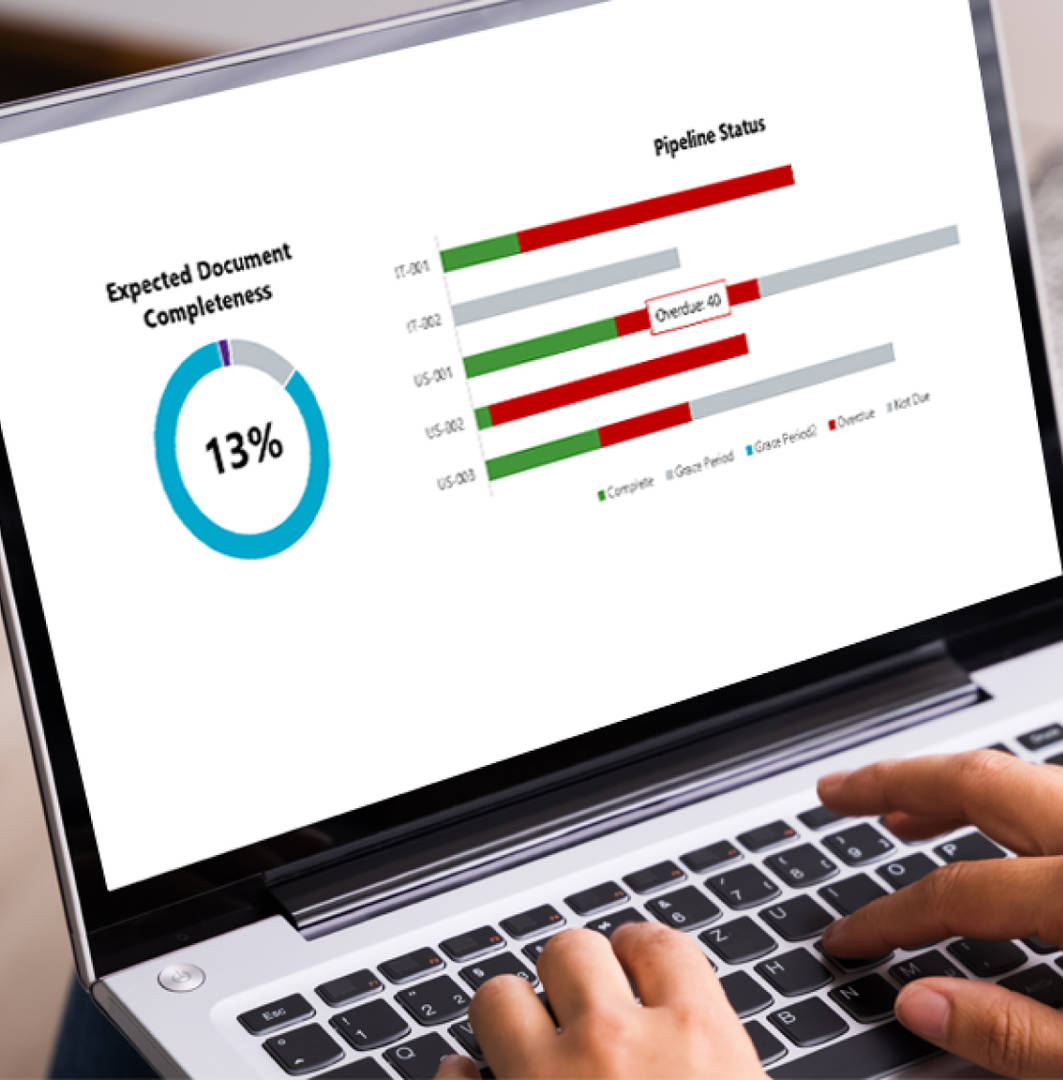
Cencora TMF heatmaps provide a detailed visual and textual roadmap to improving TMF health and inspection-readiness.
TMF heatmaps

Cencora TMF heatmaps provide a detailed visual and textual roadmap to improving TMF health and inspection-readiness.
The TMF heatmaps solution is extremely helpful for shining a light into legacy studies, where the quality of the TMF may be completely unknown. In addition, our customers find our heatmaps to be an excellent management tool for ongoing studies, demonstrating clear oversight and understanding of the study at key milestones such as study startup, prior to a regulatory submission, and so forth.
Our TMF heatmaps solution provides a snapshot, from any eTMF system, of where documents may be missing across a study. This visual overview and analysis makes it easy to pinpoint areas requiring further review or remediation, enabling a much more efficient risk-based quality review approach.
Our TMF heatmaps solution provides a snapshot, from any eTMF system, of where documents may be missing across a study. This visual overview and analysis makes it easy to pinpoint areas requiring further review or remediation, enabling a much more efficient risk-based quality review approach.

Cencora TMF heatmaps provide a detailed visual and textual roadmap to improving TMF health and inspection-readiness.
Connect with our TMF experts to learn more
Get in touch to learn about how our solutions can help accelerate better health outcomes. Whether you have questions or just need more information, our experts are here to assist you.
At Cencora, we understand that effective Trial Master File (TMF) management is a cornerstone of successful clinical trial execution, regulatory inspection readiness, and long-term document governance. As a global provider of TMF services, we are fully committed to ensuring that all personal data processed within our TMF platforms and services complies with applicable privacy and data protection laws worldwide. TMF Solutions Global Privacy Statement