Exploring cell and gene therapy logistics and modalities
Updated for 2024 Q3

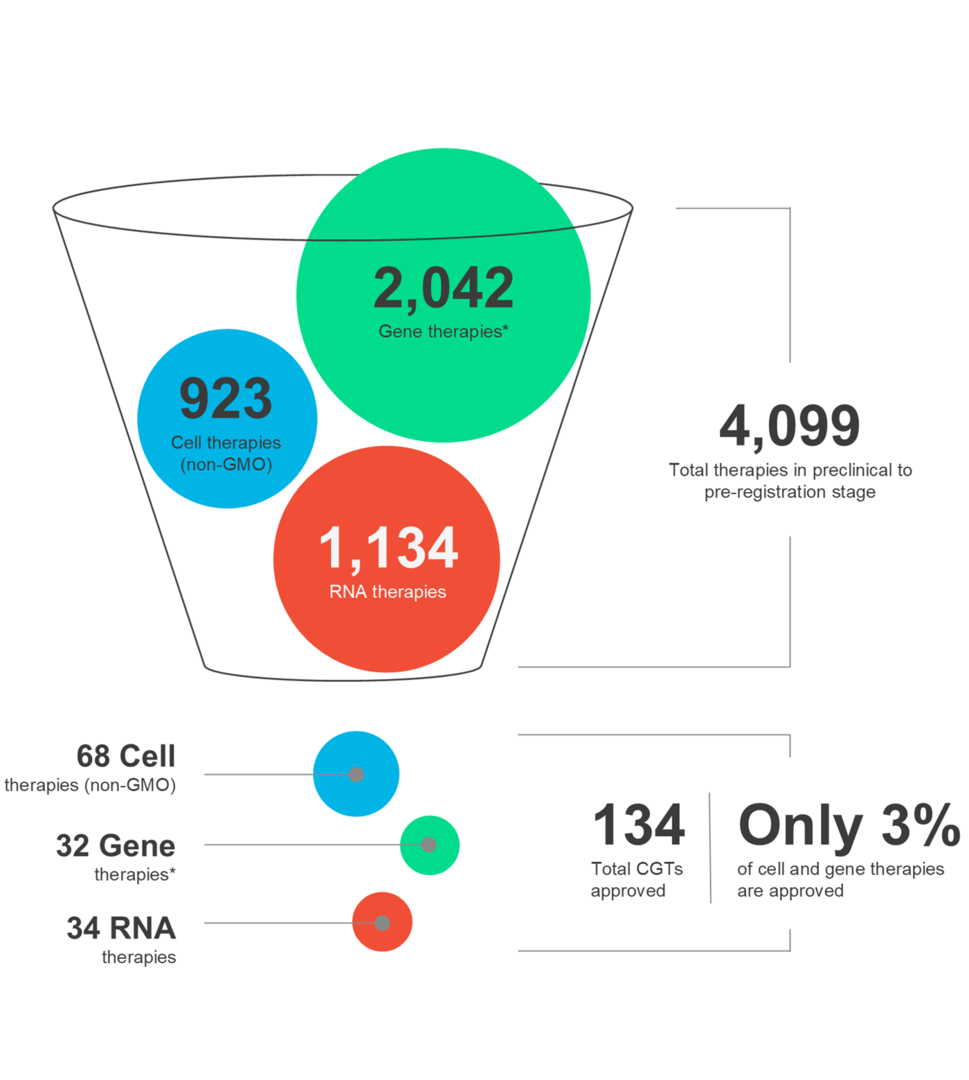
Cell and gene therapies (CGTs) are shaping the future of medicine and offering new hope to patients worldwide. These advanced therapies possess the power to target a wide range of conditions. However, it is crucial to recognize that the CGT landscape is dynamic and therapeutic success requires careful consideration of multiple factors throughout the entire drug development and distribution process.
In our latest infographic, explore the latest market trends and the nuances of various CGT modalities, along with autologous and allogeneic therapies.
*Available in English only

Cell therapy development pipeline FAQs
Which cell therapies are approved?
There are many approved cell therapies, some examples include:
- Kymirah (tisagenlecleucel): A type of immunotherapy known as CAR-T cell therapy and it's used to treat certain types of non-Hodgkin lymphoma and acute lymphoblastic leukemia
- Yescarta (axicabtagene ciloleucel): A CAR-T cell therapy used for treating large B-cell lymphomas after two or more lines of systemic therapy
- Provenge (sipuleucel-T): An autologous cellular immunotherapy indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer
What is the cell therapy approval process?
Cell therapy approval involves a rigorous process overseen by regulatory bodies like the food and Drug Administration (FDA). It begins with preclinical testing in labs and animal models, followed by an investigational new drug (IND) application. If approved, clinical trials are conducted in three phases to assess safety, efficacy, and dosage. Post-trial, a Biologics License Application (BLA) is submitted for review. If successful, the therapy is approved for market but continues to be monitored for long-term effects.
How many approved cell therapies are there?
The number of approved cell therapies can vary by country, as each has its own regulatory bodies and approval processes. In the U.S., cell therapies are regulated by the FDA, while in Europe they fall under the purview of the European Medicines Agency. These agencies have different criteria and timelines for approval. Furthermore, with advancements in medical research and technology, new cell therapies are being developed and tested continually. Consequently, the number of approved cell therapies is steadily growing worldwide.




